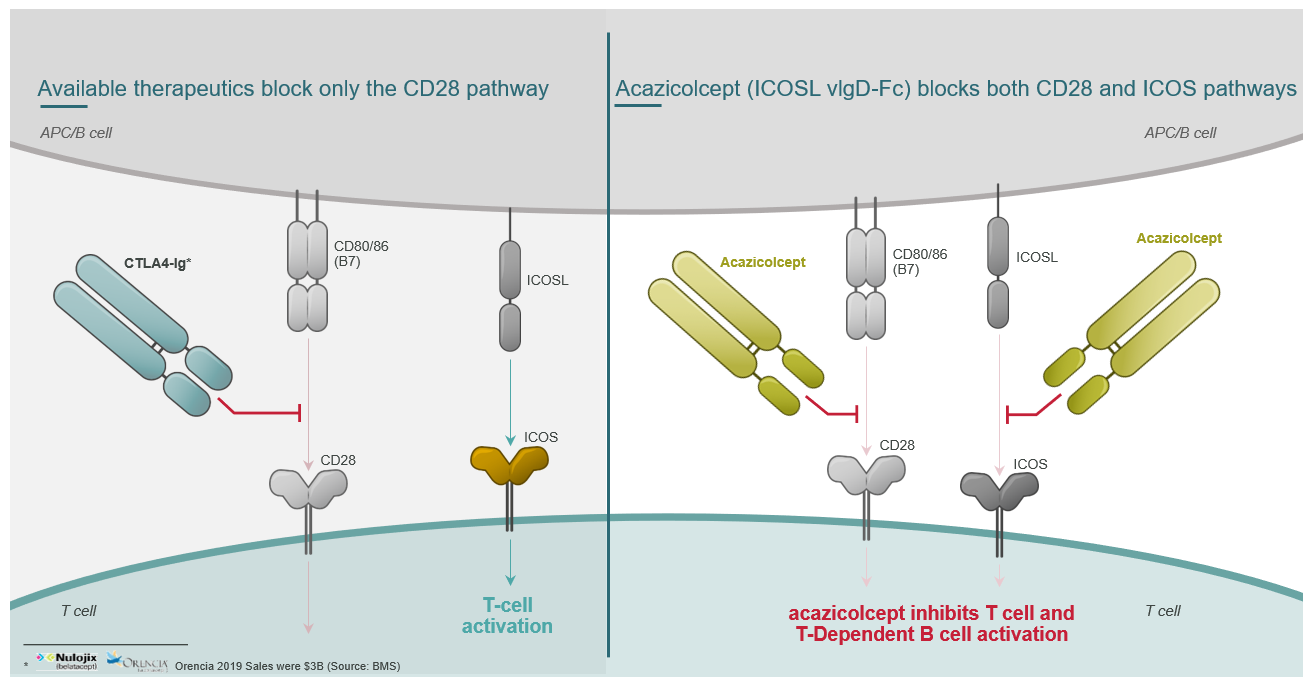
Acazicolcept is a first-in-class, dual inhibitor of the CD28 and ICOS T-cell costimulatory pathways being developed for treatment of systemic lupus erythematosus (SLE). By simultaneously blocking two key costimulatory pathways, acazicolcept has the potential to improve outcomes in patients suffering from severe autoimmune/inflammatory diseases. Preclinical studies have demonstrated efficacy in models of SLE, Sjögren’s syndrome, arthritis, inflammatory bowel disease, multiple sclerosis, type 1 diabetes, uveitis, and graft versus host disease.
Synergy (NCT04835441) is a global, randomized, double-blind, placebo-controlled phase 2 clinical study of acazicolcept in moderate-to-severe SLE that initiated enrollment in June 2021.
In December 2023, we announced an amendment to the previously announced 2020 option and license agreement with AbbVie for acazicolcept.
For full details, please see here.